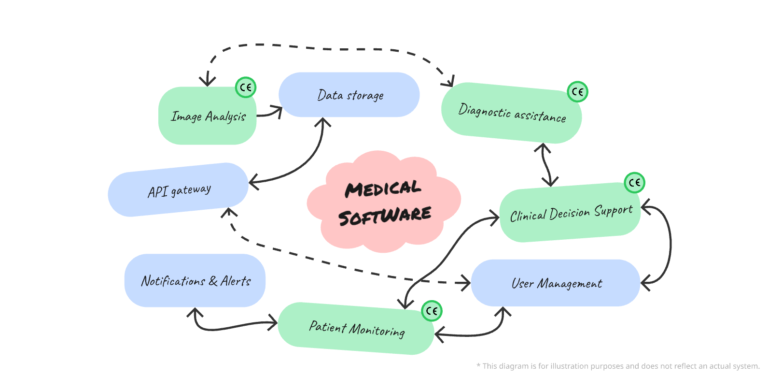
Medical device feature management
Obtaining the CE marking for software as a medical device (MD) is a crucial step for market access in Europe. However, not all software features are intended for medical purposes. Distinguishing between MD and non-MD features is essential to ensure compliance with the requirements of Regulation (EU) 2017/745 (MDR) and to maintain accurate regulatory documentation….